Sevuparin
Sevuparin, a heparinoid, has been designed to retain its inflammation modifying properties while causing significantly less blood-thinning. As a result, sevuparin can be dosed at significantly higher levels than other comparable heparinoids, allowing it to be used to treat multiple diseases that are caused by severe inflammation.
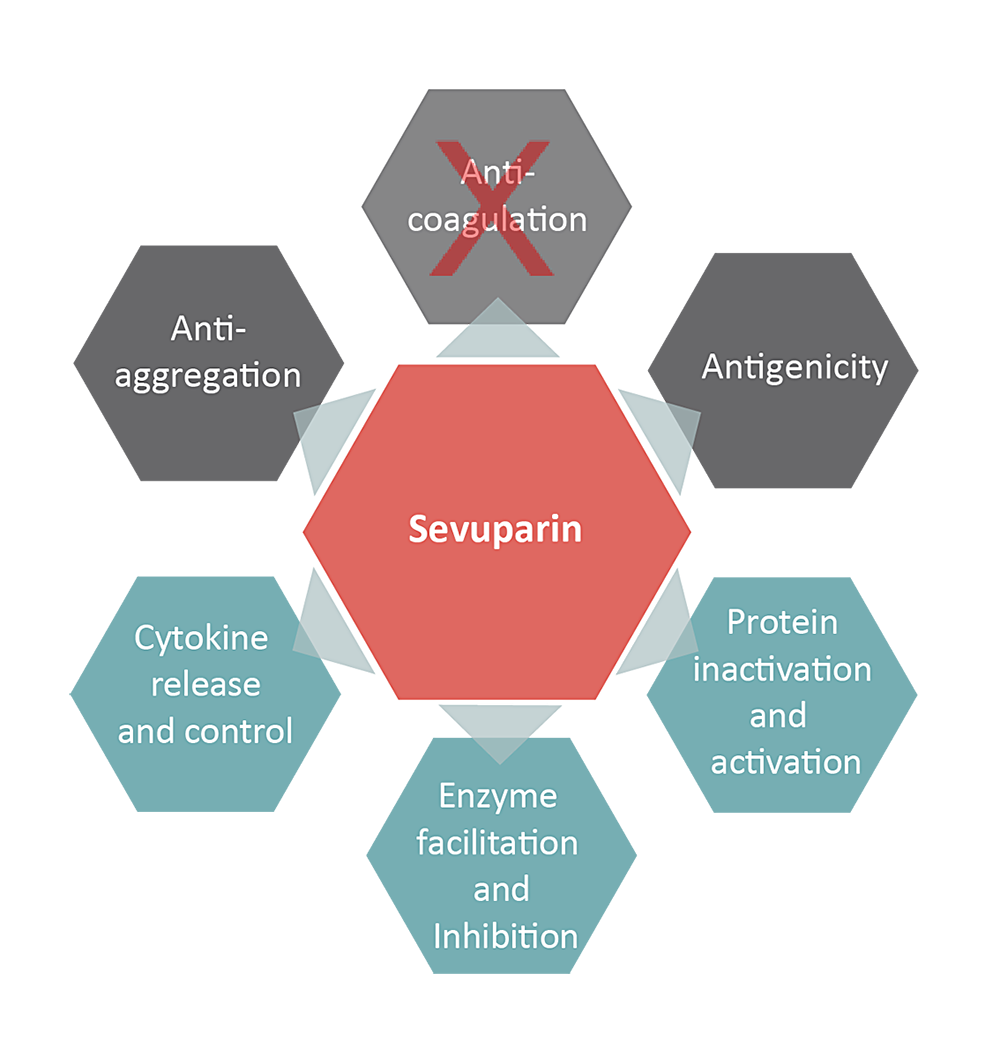
Thanks to its unique properties and a confirmed safety profile, sevuparin has the potential to greatly improve the treatment of sepsis/septic shock and other conditions with acute systemic inflammation for example severe trauma, burns, major surgery, and severe malaria. Furthermore, the properties of sevuparin could also address states of anemia that are related to chronic inflammatory diseases such as kidney disease.
Sevuparin has been approved for clinical research in both US and Europe. Clinical experience with sevuparin in >200 healthy volunteers and patients with malaria or sickle cell disease has already confirmed the product’s strong safety profile.
Two routes of administration of sevuparin are currently being developed – an IV formulation for in-patient administration and a subcutaneous formulation that allows ambulatory and home care administration.
